Your Time has Finished
Loading...
BMAT 2015-S2
Your Score: %
Average Score of All Users:
You performed better than of students
Section Breakdown
| Your Score | Average of all Users | Percentile | |
|---|---|---|---|
| BMAT 2015 S2 |
BMAT 2015 S2
Your score:
Average score:
You performed better than of students
This section is Section 2 of 3.
Speed as well as accuracy is important in this section. Work quickly, or you might not finish the paper. There are no penalties for incorrect responses, only marks for correct answers, so you should attempt all 27 questions. Each question is worth one mark.
You must complete the answers within the time limit. Calculators are NOT permitted.
Good Luck!
Note – if press “End Exam” you can access full worked solutions for all past paper questions
Which one of the following is not part of a reflex to the stimulus of placing your hand on a hot object?
Explanation
The correct answer is E.
The brain does not take part in the autonomic nervous system. The response to touching a hot plate, gives an involuntary and reflex response. The brain is not involved in such responses, as they are unconscious and involuntary. The electrical impulse is passed onto the relay neurone, after the sensory neurone, across a synapse.
Which of the following organic compounds will decolourise bromine water by reacting with it at room temperature?
1 C₄H₄
2 Polypropene
3 CH₂C(CH₃)₂
4 CH₃CH₂I
Explanation
The correct answer is C.
Alkenes (the presence of a C=C bond) causes bromine water to change colour from orange to colourless. 1) is ethene, an alkene, so would decolourise bromine water. 2) is a polymer, which is formed from many propene monomers joined together to form a saturated compound, so does not contain any C=C bonds and will not decolourise bromine water. 3) is 2-methylprop-1-ene, which is another alkene so will decolourise bromine water. 4) is a haloalkane so will not decolourise bromine water. Therefore only compounds 1 and 3 will decolourise bromine water.
 Preem
Medicmind Tutor
Preem
Medicmind Tutor
Tue, 17 Aug 2021 09:23:03
the first choice chose be C2H4 instead of C4H4
 s
Medicmind Tutor
s
Medicmind Tutor
Tue, 28 Jun 2022 09:21:58
yeah but you get the point that is unsaturated
The colour of the surface of an object has an effect on the rate of infrared radiation that it absorbs and emits. As a result of this, some clothes which are otherwise identical will keep a person warmer when outside in winter because of their differences in colour.
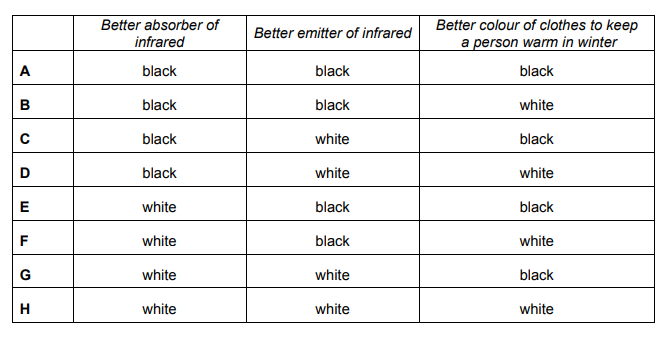
Explanation
The correct answer is B.
Black coated surfaces are the best absorbers and emitters of infrared radiation. In order to keep a person warm in the winter, infrared radiation needs to be prevented from being emitted from the person. White is the worst emitter and absorber of infrared radiation, and so restricts heat lost.
A bag contains only 8 beads.
The beads are identical in all respects except colour.
3 of the beads are black and the other 5 beads are white.
A bead is taken at random from the bag and not replaced.
A second bead is then taken at random from the bag.
Explanation
The correct answer is B.
The probability of getting a black in the first selection, is 3/8. As the ball is not replaced, the sample size: 7. Hence, the probability of getting a black ball in the second selection is 2/7. The probability of getting 2 black balls, BB, is 3/8 X 2/7 = 6/56 = 3/28.
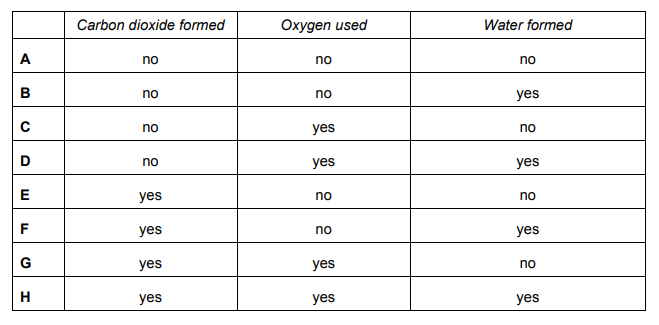
Explanation
The correct answer is A.
Anaerobic respiration occurs in the absence of oxygen – when insufficient oxygen is available for aerobic respiration to occur.
Hence, glucose is broken down to lactic acid; no carbon dioxide nor water is produced.
 Latisha Powers
Medicmind Tutor
Latisha Powers
Medicmind Tutor
Fri, 29 Oct 2021 17:52:51
I’m a bit confused on what the products of anaerobic respiration are. In a previous video, it was mentioned that H2O is a product of anaerobic respiration but it doesn’t say that here. Also, if you Google it, CO2 is also a product.
 Black lightning
Medicmind Tutor
Black lightning
Medicmind Tutor
Sun, 31 Oct 2021 16:47:59
I think its because the actual process of anaerobic respiration is just Glucose->lactic acid (+ energy.) The lactic acid gets converted to CO2 and H2O, but that isnt part of the actual process of respiration.
 aria
Medicmind Tutor
aria
Medicmind Tutor
Wed, 06 Sep 2023 12:11:16
but CO2 is produced in anaerobic respiration
 KoK0
Medicmind Tutor
KoK0
Medicmind Tutor
Sat, 14 Oct 2023 14:41:55
@aria CO2 isn't produced in anaerobic respiration
The diagram shows the suggested changes in energy labelled as a, b, c, d and e, during a chemical change from reactants to products.
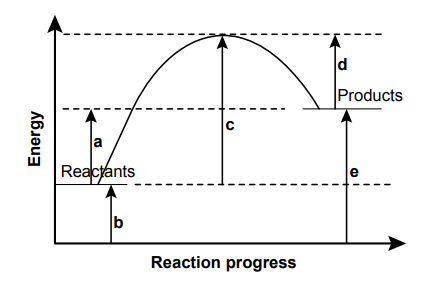
Explanation
The correct answer is A.
The energy change for the forward reaction would be the difference from the reactants to the products, which is a. The magnitude of the energy change for the reverse reaction would be the same but the sign would reverse, making the energy change of the reverse reaction -a.
A 100% efficient step-down transformer decreases the voltage of an alternating current (a.c.) electricity supply.
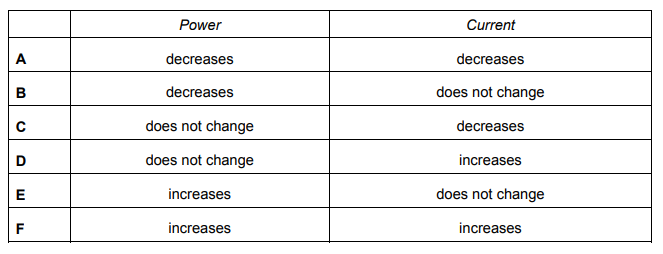
Explanation
The correct answer is D.
P=VI; as the voltage decreases, the current is increased. Hence, as the voltage decreases and the current increases, the power must remain the same.
 alainah
Medicmind Tutor
alainah
Medicmind Tutor
Mon, 10 Oct 2022 22:12:37
ahh i see thank you!
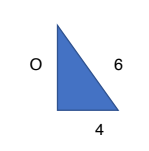
PQR is an isosceles triangle in which PQ = PR =6cm and QR =8cm.
Explanation
The correct answer is E.
As PQR is an isosceles triangle, we can split the shape into 2 right angled triangles. PQ=PR=6cm is the hypotenuse of both triangles, and QR is split into the base of 4cm each. As Tan θ= Opposite/Adjacent, we already have the adjacent length of 4cm. As we have the hypotenuse too, we can use the Pythagorean Theorem: √(62-42)=√20 = 2√5
Tan θ= Opposite/Adjacent à tan θ = 2√5/4 = √5/2
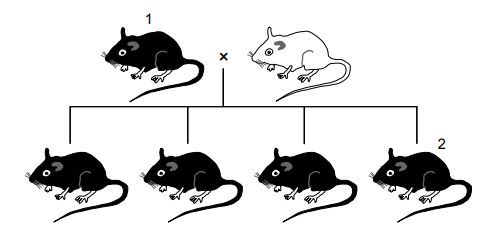
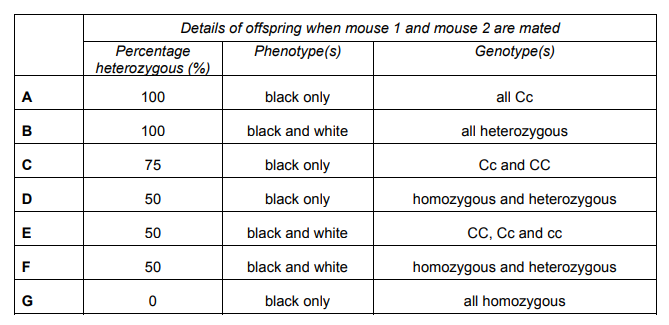
The diagram shows the results of a breeding experiment using a homozygous black mouse and a white mouse.
Explanation
The correct answer is D.
As the offspring of the first breeding experiment are all black, and it is known that Mouse 1 is homozygous, the white mouse has to be homozygous recessive for the white colour to be expressed. The offspring are all heterozygous dominant black, Cc.
| C | C | |
|
c |
Cc |
Cc |
| c | Cc |
Cc |
First breeding experiment
| C | C | |
| C | CC |
CC |
|
c |
Cc |
Cc |
Second breeding experiment
When Mouse 1 (CC) is bred with Mouse 2 (Cc), the offspring produced are all black as they all have the dominant C. Half (50%) of the offspring are heterozygous dominant (Cc) and 50% are homozygous dominant (CC). Hence, there are 2 genotypes in the population of offspring: homozygous and heterozygous.
 Junpyo Kim
Medicmind Tutor
Junpyo Kim
Medicmind Tutor
Sat, 23 Oct 2021 03:24:22
There are two scenarios possible for this question. 1. When 1 is considered CC. 2. When 1 is considered cc. According to the 1st scenario, the answer is D. According to the 2nd scenario, the answer is G.
Which of the following statements about the alkali metal rubidium is correct?
Explanation
The correct answer is D.
A Rubidium and chlorine are formed when an aqueous solution of rubidium chloride is electrolysed. – This statement is too vague, as it does not specify whether the electrodes are inert or of a suitable metal. If the metal is Rubidium, Rubidium will form. However, if it’s a metal of higher reactivity, i.e. Francium or Caesium, they will form.
B Rubidium has higher melting and boiling points than sodium. – This is incorrect, as Rubidium has lower melting and boiling points than sodium. This is as, when moving down the group 1 elements, the atomic size increases, leading to the decrease of nuclear force of attraction. This is as, there are more shells shielding the nuclear attraction from the outermost electron, which is lost more easily for delocalisation. Hence, less energy is required to overcome the attraction between the loosely bound electrons and rubidium ions. Hence, the boiling and melting points are low.
C Rubidium reacts more slowly with water than sodium, forming hydrogen gas. – This is incorrect, as rubidium reacts more vigorously, violently and almost instantaneously in water. It does form hydrogen gas; it’s more reactive than sodium.
D Rubidium is stored under oil. – This is correct, as exposure to air causes instantaneous ignition, forming rubidium oxide and oxygen. To prevent this, it is stored under oil, as with most group 1 alkaline metals.
E Rubidium sulfate has the formula RbSO₄ – Rubidium is a group 1 element, hence it has only 1 electron in its outermost shell. When ionised, it forms Rb+ Whereas, the charge on SO₄ is -2: SO₄2-. Hence, the formula is rather: Rb2SO₄
Below are three statements about radioactivity or nuclear energy.
1 Neutrons emitted in nuclear fission can cause further fission.
2 The half-life of a radioactive substance is half the time taken for all its nuclei to decay.
3 The process that produces heat and light in the Sun is called nuclear fission.
Explanation
The correct answer is A.
Neutrons emitted in nuclear fission can cause further fission, as they can be absorbed by more fission fragments, causing them to split up further. Statement 2 is incorrect – the half-life = the time taken for half of the nuclei to decay. Statement 3 is incorrect – nuclear fusion occurs in the Sun, not nuclear fission.
Given that α = 3 / 5+x , b = 3+x / 5 and c = 3+x / 5+x, where ܺ is a whole number greater than zero, which one of the following is true?
Explanation
The correct answer is B.
a<c<b
Comparing a and c first: a<c:
3 / 5+X < 3+X / 5+X
This statement is true – as the denominators are the same, only the numerators need to be compared. 3<3+X is always true, as X>0 and the addition of 3 with a whole number greater than 0 is always greater than 3.
Comparing b and c:
3+X / 5+X < 3+X / 5
This statement is also true – as the numerators are the same, only the denominators need to be compared. However, in this case, the smaller the denominator, the greater the value of the fraction (quotient rule: For the same numerator, a larger denominator will give a smaller value). 5+X is always greater than 5, as X>0. Hence, the c<b is true. As c<b is true, a<b is also true.
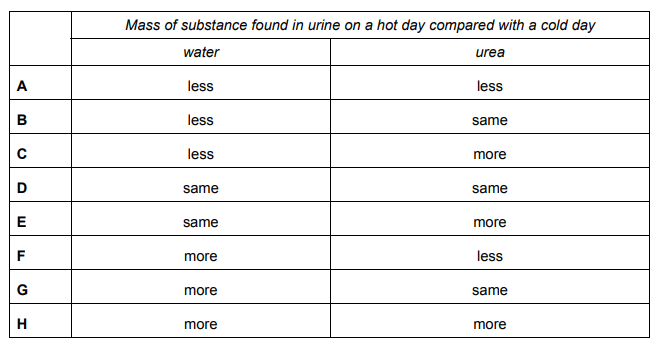
A human consumes the same amount and type of food and drink on two consecutive days. He also does the same activities on both days. However, one of the days is hot and the other is cold.
Explanation
The correct answer is B.
 Iman
Medicmind Tutor
Iman
Medicmind Tutor
Fri, 30 Dec 2022 23:31:57
But some urea is secreted with sweat, therefore there will be less urea in urine, no?
 koko
Medicmind Tutor
koko
Medicmind Tutor
Mon, 17 Jul 2023 14:27:41
what is the explanation for this pls
 Amir Khan
Medicmind Tutor
Amir Khan
Medicmind Tutor
Sat, 14 Oct 2023 14:19:01
No Explanation??!!!
Cycloalkanes are alkane compounds that are in a ring shape.
Some examples of cycloalkanes are given below.
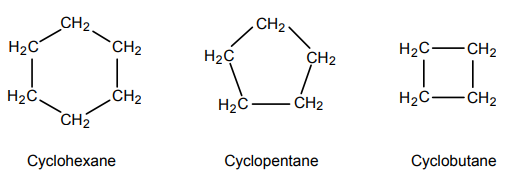
Explanation
The correct answer is C.
A They have the general formula CnH2n+2 – This is incorrect, as although they are alkanes, they are cyclic. Alkanes obey the formula CnH2n+2, whereas cycloalkanes obey CnH2n.
B They react rapidly with bromine water – This is incorrect, as alkenes react with bromine water, through electrophilic substitution, due to being unsaturated compounds, containing a double C=C bond. However, cycloalkanes are saturated compounds, with only single C-C bonds.
C They are saturated compounds – This is correct, as cycloalkanes are saturated compounds, with only single C-C bonds.
D They burn in excess oxygen to form CO2 and H2 – This is partially correct. The combustion of cycloalkanes and alkanes forms CO2 and H2O – not H2.
E They are not members of a homologous series – This is incorrect. All alkenes, alkanes and cycloalkanes are members of a homologous series. This is as, they have almost same chemical properties due to presence of same functional group (CH2). The physical properties (i.e. solubility, melting point, boiling point, state) of members of a homologous series either gradually increases or decrease with increase in molecular mass. This increase in molecular mass is uniform, often an increase in a multiple of CH2.
F They are giant covalent compounds – This is incorrect, as they are simple molecular compounds. They are held together by strong covalent bonds, yet there exist weak intermolecular forces between the molecules.
The diagram shows the only four forces acting on a model aircraft of mass 2.0kg whilst flying.
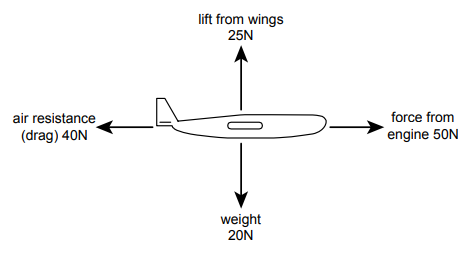
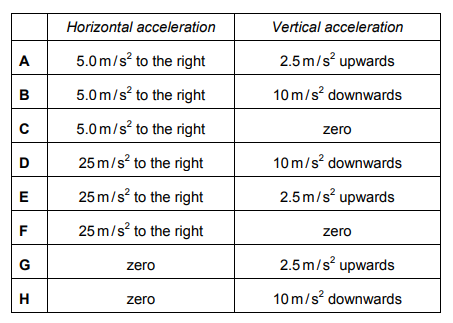
Explanation
The correct answer is A.
Horizontal acceleration: Resultant force 50N – 40N = 10N
a = F/m 10/2 = 5 ms-²
Vertical acceleration: Resultant force 25N – 20N = 5N UPWARDS
a = F/m 5/2 = 2.5 ms-²
A city football club collected money for charity at all of its matches for a year. At the end of the year the total collected was divided among three charities, A, B and C, in the ratio 1 : 2/3 : 4/5. Charity C received £3 000.
Explanation
The correct answer is E.
Multiply the ratio by the lowest common multiple, to eliminate denominators. The lowest common multiple of 1,3 and 5 is 15.
Hence, 1 : 2/3 : 4/5
15: 10: 12
Compare this with A:B:3000
The multiplying factor is, 3000/12 = 250
10 X 250 = 2500
15 X 250 = 3750
Hence, 3750: 2500: 3000
Adding them up: 3750 + 2500 + 3000 = 9250
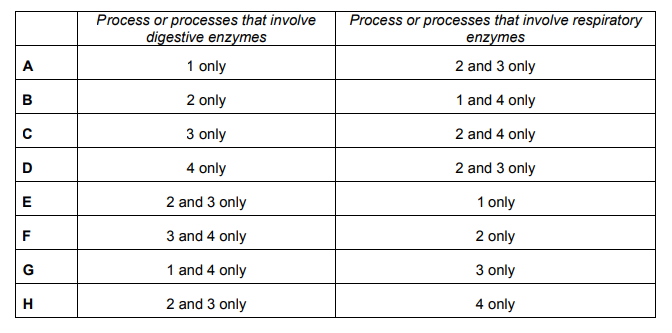
The diagram shows part of the carbon cycle.
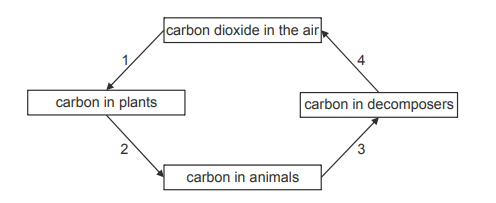
Explanation
The correct answer is H.
Firstly, digestive enzymes are used in Process 2. This is when plants animals eat plants, and so digestive enzymes break down long chains of carbon compounds into their smaller organic forms (fatty acids, glycerol, amino acids, glucose etc), to be assimilated in the body as fat, muscle etc. When animals die, they are decomposed by saprobiotic decomposers, which are microorganisms that release digestive enzymes. These enzymes catalyse the breakdown of organic compounds that have been ingested by the animals, thus releasing carbon containing compounds into the soil. Respiratory enzymes are only used in Process 4, which is respiration. Saprobiotic decomposers respire aerobically as they assimilate the products of digestion, thus releasing carbon dioxide into the atmosphere.
The reaction between the gases S and T occurs according to the following equation.
S(g) + 2T(g) → W(g) + 2X(g) ∆H = -12 kJmol–1
Explanation
The correct answer is C.
A is incorrect as catalysts increase the rate of reaction. B is incorrect as gases react faster than solids. C is correct because it would take longer for more molecules to have energy equal to or greater than a higher activation energy compared to a lower one. D is incorrect as increasing the temperature gives reacting particles more kinetic energy and means more will have at least the activation energy and there will be an increased frequency of successful collisions between particles, so the rate would increase. E is incorrect because there are an equal number of moles of gas in the reactants as in the products, and the same number of moles of any ideal gas occupies the same volume, so there would be no change in gas volume.
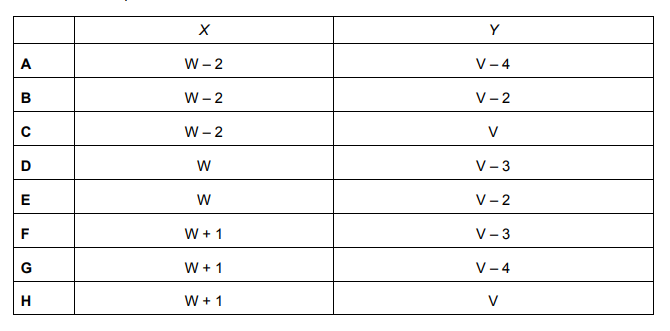
Part of a radioactivity decay series is represented below. It involves the change of a nucleus M into a nucleus N by the emission of a beta particle, followed by a further change into a nucleus Q by the emission of an alpha particle. Four quantities V, W, X and Y are shown.
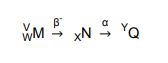
Explanation
The correct answer is G.
When a beta particle (electron) is emitted, a neutron turns into a proton, hence the atomic number (proton number) increases, hence W (atomic number of M) + 1 = X (atomic number of X). When an alpha particle is emitted, 2 protons and 2 neutrons are released. Hence, the mass number decreases by 4. (Y = V – 4).
A class of n pupils takes a spelling test. Their mean score for the test is m. Another pupil takes the test and scores n. When this pupil’s result is included with the other results it is found that the mean has decreased by 2.
Explanation
The correct answer is D.
Let X = total score.
Mean Score = Total Score/Number of pupils → m = x/n → mn = x
Inclusion of another pupil’s test score: m-2 = x+n/n+1
For every X substitute mn= x
m-2 = mn+n/n+1(multiply both sides by (n+1) to get rid of denominator)
(m-2)(n + 1)=mn+n
Expand brackets on Left Hand Side: mn – 2n + m – 2 = mn + n
As we want to express in terms of n, rearrange equation with all ‘n’s on one side:
3n=m-2
Divide both sides by 3: n = m-2/3
Mitochondria are the site of aerobic respiration in animal cells. A theory of the evolution of animal cells states that these mitochondria may once have been aerobic bacteria that were taken into the cytoplasm of a cell in an early ancestor of the animals, allowing the cells to gain the ability to respire using oxygen.
1 The structure of their DNA is a double helix.
2 They would both possess a cell wall.
3 They would both possess a nucleus.
4 They would both possess a cell membrane.
Explanation
The correct answer is A.
Statements 1 and 4 are correct. Statement 2 is incorrect, as although aerobic bacteria (prokaryotic cells) would possess a cell wall, human white blood cells do not have a cell wall. Statement 3 is incorrect, as although a human white blood cell would contain a nucleus, prokaryotic cells do not have a nucleus – their DNA is chromosomal, with no true nucleus, commonly circular as a plasmid. Both their DNA are double helixes, and both eukaryotic and prokaryotic cells possess a cell membrane.
![]()
Explanation
The correct answer is C.
For this question, you must calculate the electron number of each ion/element given. This can be found on the atomic number. The charges – positive and negative – change the electron numbers from their elements, as these elements have been ionised. If the atom is negatively charged, add 1 electron to the atomic number, and if the atom is positively charged, subtract 1 from the atomic number.
Cl- : 17 + 1 = 18 ; Cl+ : 17 – 1 = 16 ; Ar : 18 ; K+ : 19 – 1 = 18, Ca+: 20 – 1 = 19,
K- : 19 + 1 = 20
Hence, Cl- , Ar and K+ have the same electron arrangement: 18 electrons.
A car is being driven at 20m/ s when the driver sees a child run into the road. The driver’s usual reaction time is 0.70s, but this is doubled because the driver is tired. Once the driver applies the brakes, the car is brought uniformly to rest in a further 3.3s. What is the total distance travelled by the car between when the driver first sees the child to when the car stops?
Explanation
The correct answer is D.
Distance traveled in reaction time:
s = vt
s = 20(2(0.7))
s = 28 m
Distance traveled while braking:
s = 1⁄2 (u+v)t
s = [(20 + 0)/2]x3.3
s = 33 m
Hence, Total distance:
d = 38 + 33
d = 61 m
 aria
Medicmind Tutor
aria
Medicmind Tutor
Wed, 06 Sep 2023 12:27:00
what is that formula to find distance while braking
 C
Medicmind Tutor
C
Medicmind Tutor
Tue, 03 Oct 2023 19:06:18
S=ut^2+1/2at^2
Simplify:
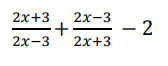
Explanation
The correct answer is D.
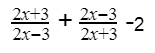
Lowest common multiple of all 3 fractions is: (2x− 3)(2x + 3) Hence, rewrite equation, using cross multiplication:
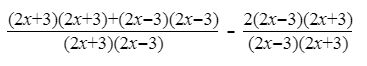
Simplify the numerators:
(2x+3)2= 4x2+ 12x + 9
(2x-3)2 =4x2− 12x + 9
Hence, (2x+3)2+(2x-3)2= 8x2+ 18
2(2x − 3)(2x + 3) = 8x2− 18
8x2 + 18 −(8x2− 18) = 8x2+ 18 −8x2+ 18 = 36
Hence, 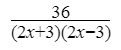
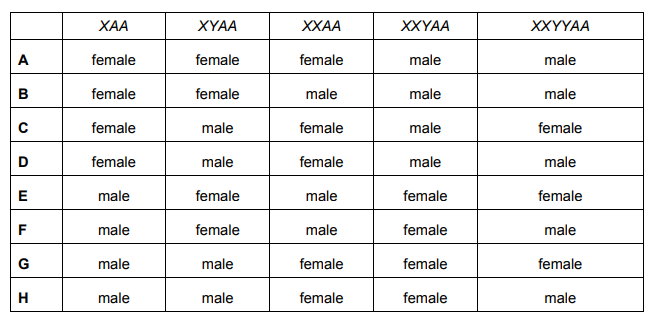
The sex of a species of fruit fly is determined by the number of X chromosomes relative to the number of non-sex chromosomes (A) in a cell. This is called the X : A ratio.
A fruit fly will be male if X : A = 0.5:1 and female if X : A = 1 : 1.
The Y chromosome contains genes necessary for making sperm.
Explanation
The correct answer is G.
A fruit fly will be male if X: A = 0.5:1 and female if X: A = 1: 1. Here, the determination of sex is clearly stated by the X:A ratio. Although the Y chromosome contains genes for sperm production, the determination of sex is only controlled by the X:A ratio. The genes for sperm production can be activated or be dormant, depending on the X:A ratio. Hence, the presence of a Y chromosome has no effect on sex determination.
XAA – 1X:2A (1:2 ratio), 0.5:1 = 1:2 hence male
XYAA – 1:2 ratio (no effect of Y) = male
XXAA – 2X:2X (2:2 ratio) = female
XXYAA – 2:2 ratio (no effect of Y) = female
XXYYAA – 2:2 ratio (no effect of 2Y either) = female
The equation for the complete combustion of methane is:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
(Ar: H = 1 ; C = 12 ; O = 16)
Explanation
The correct answer is B.
To answer this question, firstly, work out the moles on the CH₄.
n(CH₄) = m/Mr = 1.6/(12+4)= 0.1
CH₄:2H₂O – ratio of 2:1 (compare these ratios, as the mass of water is unknown)
Hence, moles of water = 2 x 0.1 = 0.2
Mass of water = n x Mr(water) = 0.2 x 18 = 3.6
Form an equation of mass, where (8-x) represents the quantity of reacted oxygen; x = unreacted oxygen
Hence: 1.6 + (8 – x) = 4.4 + 3.6
1.6 + 8 – x = 8
Hence, x = 1.60g.
Consider the following three statements:
1 A mass of 4.0kg requires a resultant force of 5.0N to act on it in order to accelerate it at 1.25 m/s².
2 A wave of frequency 4.0Hz travelling at a speed of 5.0m/ s has a wavelength of 1.25m.
3 A voltage of 4.0V applied across a 5.0Ω resistor causes a current of 1.25A to flow.
Explanation
The correct answer is D.
Statement 1 is correct – F = ma 4 x 1.25 = 5
Statement 2 is correct – c= f� ; 5 = 4 x 1.25
Statement 3 is incorrect – V = IR, As 4 NOT EQUAL to 5 x 1.25
BMAT 2015 S2 Review Screen
Instructions
Below is a summary of your answers. You can review your questions in three (3) different ways.
The buttons in the lower right-hand corner correspond to these choices:
1. Review all of your questions and answers.
2. Review questions that are incomplete.
3. Review questions that are flagged for review. (Click the 'flag' icon to change the flag for review status.)
You may also click on a question number to link directly to its location in the exam.
BMAT 2015 S2 Section
Final Answer Review Screen
Instructions
This review section allows you to view the answers you made and see whether they were correct or not. Each question accessed from this screen has an 'Explain Answer' button in the top left hand side. By clicking on this you will obtain an explanation as to the correct answer.
At the bottom of this screen you can choose to 'Review All' answers, 'Review Incorrect' answers or 'Review Flagged' answers. Alternatively you can go to specific questions by opening up any of the sub-tests below.
BMAT 2015 S2 Section
TI-108